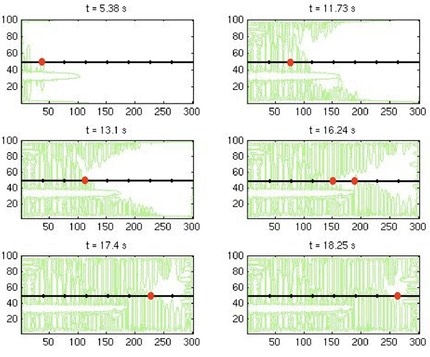
A famous experiment by Richard Helmuth* showed that ice grows through the pores of cement paste or concrete at a rate controlled by diffusion of heat. The temperature of the water/ice interface rises until it is close to the melting point, and the melting point is depressed by the small size of the crystals (15-30 nm). A mysterious feature of Helmuth’s data is that the growth rate is very fast - in fact, it is too fast to be explained by heat flow. Recent simulations by Zhenhua Sun, using a level set method, show that there is a flaw in Helmuth’s experimental design. He immersed a sample of cement paste in a cold bath, then nucleated growth of ice at one end of the sample; he followed the rate of growth by noting the time at which a series of thermocouples, embedded along the length of the sample, detected the heat released by the ice. The simulations shown above reveal that the ice grows rapidly along the surface of the sample, where it is in contact with the cold bath, then dendrites grow inward from the surface. The thermocouples (indicated by black dots) respond to the latter dendrites, not to ice advancing from the left. The actual growth rate of individual dendrites is ~1/3 as fast as the rate inferred from the response of the thermocouples. Thus, Helmuth was right about the role of heat flow control, even though his measured growth rates were an artifact.
These results will be presented at the 12th International Congress on the Chemistry of Cement in Montreal, July 8-13, 2007. A draft of the paper is available here.
To extend our study of dendritic growth within pores, Olivier Abellan is using a phase field method. Those results will be published shortly.
This work was supported by NSF Grant CMS-0509986.
*R. Helmuth, Capillary size restrictions on ice formation in hardened Portland cement pastes, in: Proc. 4th Int. Cong. Chemistry of Cement, NBS Monog. 43, Vol. II, National Bureau of Standards, Washington, D.C., 1962, pp. 855-869