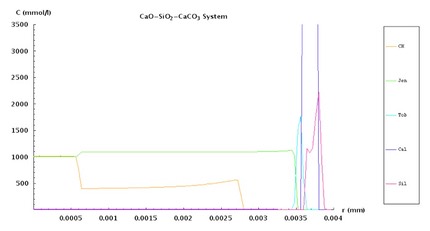
 This is a simulation of the changing phases within a cylinder of cement paste (4 mm radius) that is attacked by carbonic acid, as in the experiments performed by Andrew Duguid. In this animation, the Calcium Aluminate phases (CaO-Al2O3-CaSO4-CaCl2-H2O system) in the cement are shown. Two different mechanisms are seen:
This is a simulation of the changing phases within a cylinder of cement paste (4 mm radius) that is attacked by carbonic acid, as in the experiments performed by Andrew Duguid. In this animation, the Calcium Aluminate phases (CaO-Al2O3-CaSO4-CaCl2-H2O system) in the cement are shown. Two different mechanisms are seen:
- ingress of chloride leads to monosulfoaluminate (AFm) dissolution and Friedel’s salt (FS) formation according to a reaction that involves portlandite depletion and ettringite (Ettr) formation:
- hydrolysis of cement hydrates: Calcium hydroxide (CH) first dissolves. Then, in a very narrow zone, Friedel’s salt and ettrringite successively dissolve leaving behind only an alumina gel (along with silica gel), represented here as diaspore.
Cement Corrosion:
Calcium Aluminate Hydrates